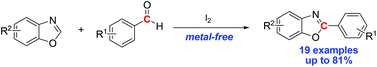
A simple and efficient methodology for the arylation of benzoxazoles with aldehydes using iodine as the mediator has been developed. The reaction proceeded smoothly with a range of substrates to give the corresponding arylated products in moderate to good yields
Green Chem., 2013, 15,2365-2368
DOI: 10.1039/C3GC41027G, Communication
DOI: 10.1039/C3GC41027G, Communication
*
Corresponding authors
a
Institute of Bioengineering and Nanotechnology, 31 Biopolis Way, The Nanos, Singapore 138669, Singapore
E-mail: ygzhang@ibn.a-star.edu.sg ;
Fax: (+65) 6478-9020
E-mail: ygzhang@ibn.a-star.edu.sg ;
Fax: (+65) 6478-9020
