Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC00932H, Paper
DOI: 10.1039/C6GC00932H, Paper
James Sherwood, Helen L. Parker, Kristof Moonen, Thomas J. Farmer, Andrew J. Hunt
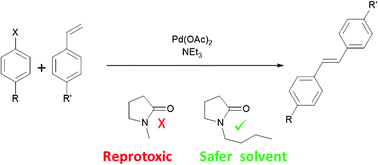
N-Butylpyrrolidinone (NBP) has been demonstrated as a suitable safer replacement solvent for N-Methylpyrrolidinone (NMP) in selected organic syntheses.
N-Butylpyrrolidinone (NBP) has been demonstrated as a suitable safer replacement solvent for N-Methylpyrrolidinone (NMP) in selected organic syntheses.
N-Butylpyrrolidinone as a dipolar aprotic solvent for organic synthesis
*Corresponding authors
aGreen Chemistry Centre of Excellence, Department of Chemistry, University of York, UK
E-mail: andrew.hunt@york.ac.uk
E-mail: andrew.hunt@york.ac.uk
bEastman Chemical Company, Pantserschipstraat 207 – B-9000, Gent, Belgium
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC00932H
Dipolar aprotic solvents such as N-methylpyrrolidinone
(or 1-methyl-2-pyrrolidone (NMP)) are under increasing pressure from
environmental regulation. NMP is a known reproductive toxin and has been
placed on the EU “Substances of Very High Concern” list. Accordingly
there is an urgent need for non-toxic alternatives to the dipolar
aprotic solvents. N-Butylpyrrolidinone, although structurally
similar to NMP, is not mutagenic or reprotoxic, yet retains many of the
characteristics of a dipolar aprotic solvent. This work introduces N-butylpyrrolidinone
as a new solvent for cross-coupling reactions and other syntheses
typically requiring a conventional dipolar aprotic solvent.
////N-Butylpyrrolidinone, dipolar aprotic solvent , organic synthesis