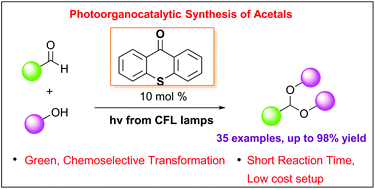
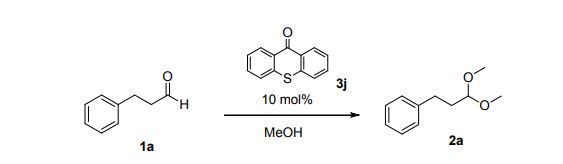
Napazimone
https://newdrugapprovals.org/2026/02/17/napazimone/
Anthony Melvin Crasto AfricurePharma Row2Tech Anax AdvectProc Niper-G
Napazimone
https://newdrugapprovals.org/2026/02/17/napazimone/
Anthony Melvin Crasto AfricurePharma Row2Tech Anax AdvectProc Niper-G