2-Methoxy-4-propylphenol
2-Methoxy-4-propylphenol
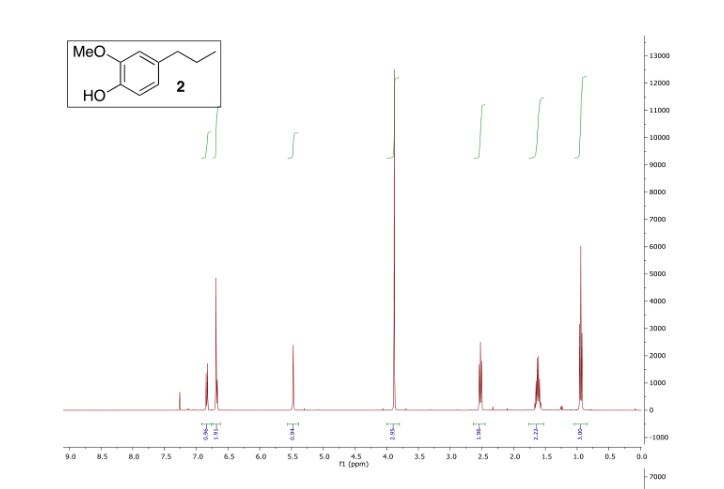
1H NMR (400 Mhz, CDCl3) δ (ppm): 6.83 (1H,d, J = 7.8 Hz), 6.68 (2H, d, J = 7.4 Hz), 5.47 (1H, s), 3.79 (3H, s), 2.52 (2H, t, J =7.6 Hz), 1.61 (2H, sext, J = 7.5 Hz) 0.94 (3H, t, J = 7.3 Hz).
1H NMR (400 Mhz, CDCl3) δ (ppm): 6.83 (1H,d, J = 7.8 Hz), 6.68 (2H, d, J = 7.4 Hz), 5.47 (1H, s), 3.79 (3H, s), 2.52 (2H, t, J =7.6 Hz), 1.61 (2H, sext, J = 7.5 Hz) 0.94 (3H, t, J = 7.3 Hz).
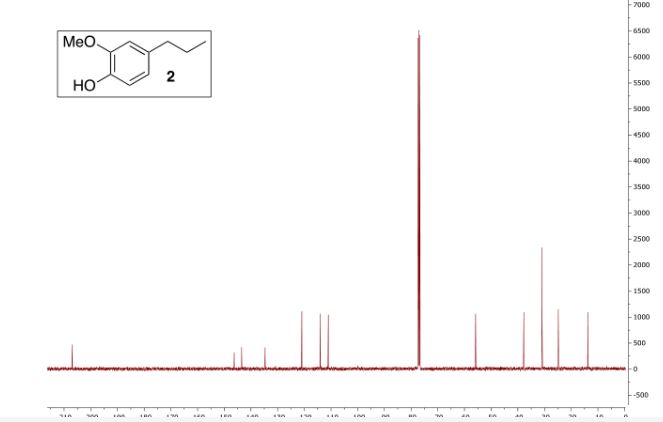
13C NMR (100 MHz, CDCl3) δ (ppm) 146.4, 143.6,134.7, 121.0, 114.2, 111.1, 55.9, 37.8, 24.9, 13.8.
HRMS (ESI-TOF) m/z: [M + H]+ calculated for C10H15O2:167.1067; found: 167.1064.
DOI: 10.1021/acs.oprd.6b00441
/////////






















