World Drug Tracker: ESCITALOPRAM:
'via Blog this'
DR ANTHONY MELVIN CRASTO Ph.D ( ICT, Mumbai) , INDIA 30 Yrs Exp. in the feld of Organic Chemistry. Serving chemists around the world. Helping them with websites on Chemistry.Millions of hits on google, world acclamation from industry, academia, drug authorities for websites, blogs and educational contributionn
Sunday, 15 September 2013
Monday, 9 September 2013
Antioxidant Mechanism of Active Ingredients Separated from Eucalyptus globulus
| Antioxidant Mechanism of Active Ingredients Separated from Eucalyptus globulus | |||
| N. M. El-Moein, E. A. Mahmoud and Emad A. Shalaby* | |||
| Biochemistry Department, Faculty of Agriculture, Cairo University, Giza, Egypt, 12613 | |||
| |||
OMICS Publishing Group | Full-text | Antioxidant Mechanism of Active Ingredients Separated from Eucalyptus globulus:
'via Blog this'
Sunday, 8 September 2013
Friday, 6 September 2013
Fullerenols: Fulfilling our Future Energy Needs
Carbon to catalyze low-carbon, hydrogen economy
read all at
Sunday, 1 September 2013
Synthesis of Tolvaptan
Synthesis of Tolvaptan
pick up all this at
YANG Chuanwei~1,MU Shuai~2,LIU Ying~3,WANG Pingbao~3,LIU Dengke~(3*)
(1.School of Pharmacy,Henan University,Kaifeng 475004;2.
School of Chemical Engineering and Technology,
Tianjin University,Tianjin 300072;3.
Tianjin Institute of Pharmaceutical Research,Tianjin 300193)
(1.School of Pharmacy,Henan University,Kaifeng 475004;2.
School of Chemical Engineering and Technology,
Tianjin University,Tianjin 300072;3.
Tianjin Institute of Pharmaceutical Research,Tianjin 300193)
Tolvaptan,a selective nonpeptide arginine vasopressin V_2 receptor antagonist,was synthesized
from 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine by acylation and reduction to give 1-(4-amino-2-methylbenzoyl) -7- chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine,which was subjected to acylation with 2-methylbenzoyl chloride and reduction with sodium borohydride with an overall yield of about 45%.
from 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine by acylation and reduction to give 1-(4-amino-2-methylbenzoyl) -7- chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine,which was subjected to acylation with 2-methylbenzoyl chloride and reduction with sodium borohydride with an overall yield of about 45%.
【Key Words】: tolvaptan arginine vasopressin V_2 receptor antagonist heart failure hyponatremia synthesis
【Fund】: 天津市科技计划项目(09ZCKFSH01300)
【CateGory Index】: R914
【Fund】: 天津市科技计划项目(09ZCKFSH01300)
【CateGory Index】: R914
CAJViewer7.0 supports all the CNKI file formats; AdobeReader only supports the PDF format.
| 【Citations】 | ||
| |||||
| |||||
| 【Co-citations】 | ||
| |||
| |||
Wednesday, 21 August 2013
Direct synthesis of hydrogen peroxide in water in a continuous trickle bed reactor optimized to maximize productivity
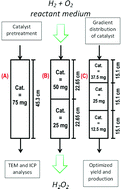
Hydrogen peroxide direct synthesis was studied in continuous mode over a 5% wt Pd/C commercial catalyst in a Trickle Bed Reactor. The target of the study was to maximize the hydrogen peroxide production. The catalyst was uniformly diluted in quartz sand at different concentrations to investigate their effects on the direct synthesis.
The amount of catalyst and the distribution of the catalyst along the bed were optimized to obtain the highest possible yield. The distribution of the catalyst along the bed gave the possibility to significantly improve the selectivity and production of hydrogen peroxide (up to 0.5% under selected conditions). Higher production rate and selectivity were found when the catalyst concentration was decreased along the bed from the top to the bottom as compared to the uniformly dispersed catalyst.
The H2/Pd ratio was found to be an important parameter that has to be investigated in the hydrogen peroxide direct synthesis. The effect of a pretreatment of the catalyst with a solution of sodium bromide and phosphoric acid was studied; the results showed how a catalyst pretreatment can lead to a real green hydrogen peroxide synthesis in water. Some optimization guidelines are also provided.
Green Chem., 2013, 15,2502-2513
DOI: 10.1039/C3GC40811F, Paper
DOI: 10.1039/C3GC40811F, Paper
*
Corresponding authors
a
Department of Chemical Engineering, Åbo Akademi University, Turku/Åbo, Finland
E-mail: bpierdom@abo.fi ;
Fax: +358 2 215 4479 ;
Tel: +358 2 215 4555
E-mail: bpierdom@abo.fi ;
Fax: +358 2 215 4479 ;
Tel: +358 2 215 4555
b
Department of Chemical Engineering and Environmental Technology, University of Valladolid, Valladolid, Spain
E-mail: jgserna@iq.uva.es
E-mail: jgserna@iq.uva.es
Subscribe to:
Comments (Atom)
