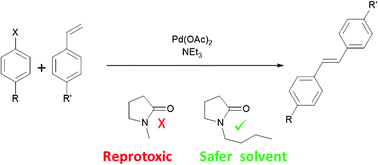
Shi et al. have reported a nickel-catalyzed decarbonylative Suzuki–Miyaura reaction which uses an N-aroylpiperidine-2,6-dione as the coupling partner for the boronic acid ( Angew. Chem., Int. Ed. 2016, 55, 6959−6963).
The method is attractive from the point of view of the stability of N-aroylpyrrolidine-2,5-diones toward storage and manipulation and the flexibility they add to the chemist’s toolbox, given their preparation from a different group of precursors to aryl halides or triflates.
Notably, the reaction uses an air-stable and inexpensive nickel catalyst, and the reactions tolerate the presence of water. While a standard reaction temperature of 150 °C is quoted, the use of temperatures as low as 80 °C also seem to be possible. Coupling efficiency is reported to be adversely affected when the aromatic rings of both of the coupling partners bear electron-donating substituents.
Ortho substituents on the aromatic rings seem to be beneficial as they facilitate decarbonylation as part of the cross-coupling. Oxidative addition into the N–C(aroyl) bond of the amide is proposed as initiating the catalytic cycle and is possible on account of a reduction in the resonance stabilization of the N-aroyl functionality versus a conventional aromatic amide.
Suzuki–Miyaura Coupling
Synthesis of Biaryls through Nickel-Catalyzed Suzuki–Miyaura Coupling of Amides by Carbon–Nitrogen Bond Cleavage (pages 6959–6963)Shicheng Shi, Guangrong Meng and Prof. Dr. Michal Szostak
Version of Record online: 21 APR 2016 | DOI: 10.1002/anie.201601914


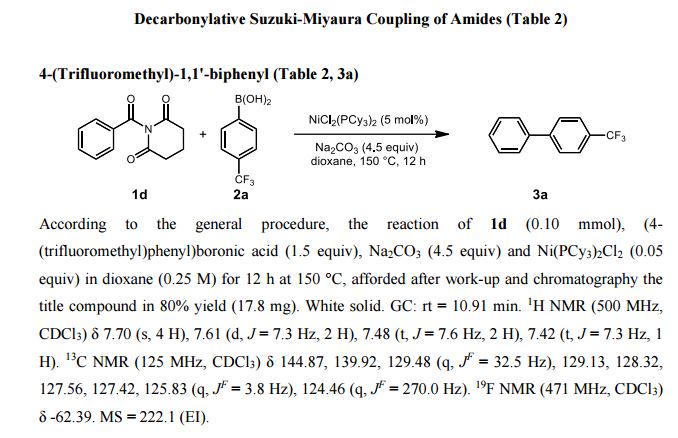
1H NMR (500 MHz, CDCl3) δ 7.70 (s, 4 H), 7.61 (d, J = 7.3 Hz, 2 H), 7.48 (t, J = 7.6 Hz, 2 H), 7.42 (t, J = 7.3 Hz, 1 H).

13C NMR (125 MHz, CDCl3) δ 144.87, 139.92, 129.48 (q, J F = 32.5 Hz), 129.13, 128.32, 127.56, 127.42, 125.83 (q, J F = 3.8 Hz), 124.46 (q, J F = 270.0 Hz).
Version of Record online: 21 APR 2016 | DOI: 10.1002/anie.201601914

Breaking and making: The first nickel-catalyzed Suzuki–Miyaura coupling of amides for the synthesis of biaryl compounds through N−C amide bond cleavage is reported. The reaction tolerates a wide range of sensitive and electronically diverse substituents on both coupling partners.

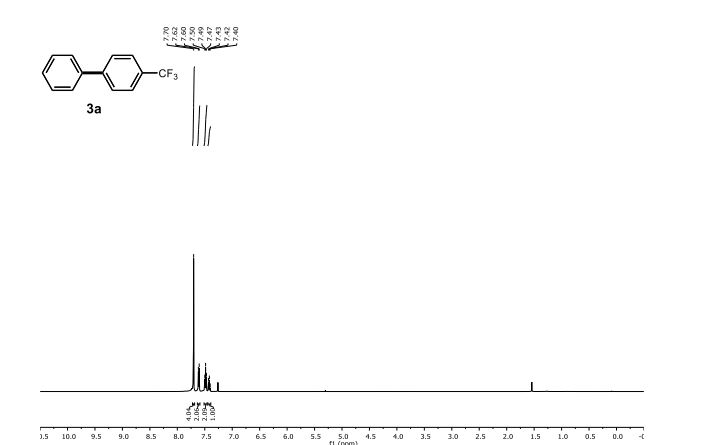
1H NMR (500 MHz, CDCl3) δ 7.70 (s, 4 H), 7.61 (d, J = 7.3 Hz, 2 H), 7.48 (t, J = 7.6 Hz, 2 H), 7.42 (t, J = 7.3 Hz, 1 H).
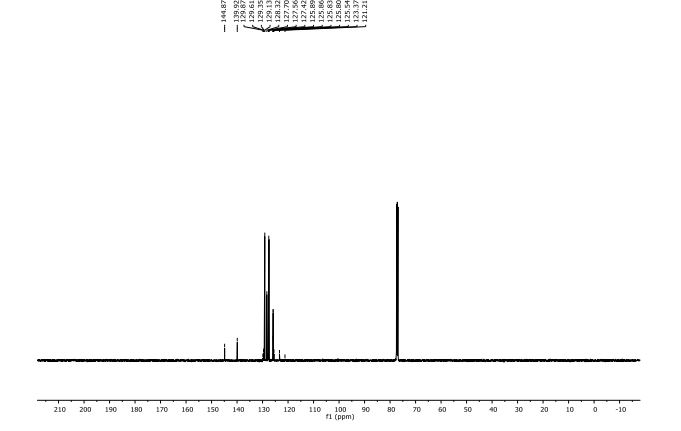
13C NMR (125 MHz, CDCl3) δ 144.87, 139.92, 129.48 (q, J F = 32.5 Hz), 129.13, 128.32, 127.56, 127.42, 125.83 (q, J F = 3.8 Hz), 124.46 (q, J F = 270.0 Hz).
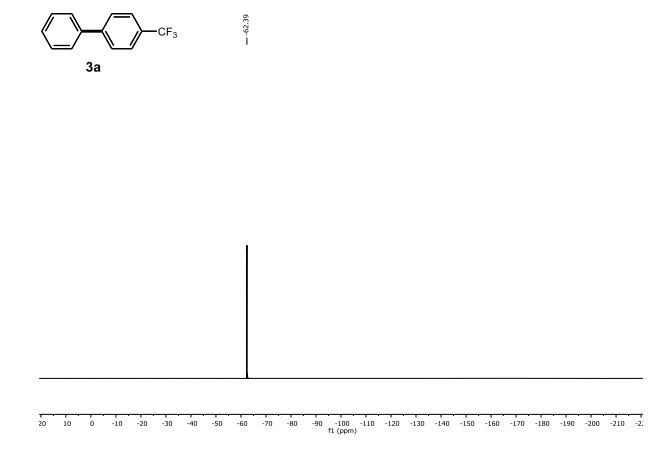
19F NMR (471 MHz, CDCl3) δ -62.39.
| Michal Szostakemail: michal.szostak@rutgers.edu office: Olson 204 |
Research Interests
The central theme of our research is synthetic organic and organometallic chemistry with a focus on the development of new synthetic methods based on transition metal catalysis and various aspects of transition metal mediated free radical chemistry and their application to the synthesis of biologically active molecules.Selected Publications
- Graphene-Catalyzed Direct Friedel-Crafts Alkylation Reactions: Mechanism, Selectivity and Synthetic Utility. Hu, F.; Patel, M.; Luo, F.; Flach, C.; Mendelsohn, R.; Garfunkel, E.; He, H.; Szostak, M. J. Am. Chem. Soc. 2015, 137 [doi]
- General Olefin Synthesis by the Palladium-Catalyzed Heck Reaction of Amides: Sterically-Controlled Chemoselective N-C Activation. Meng, G.; Szostak, M. Angew. Chem. Int. Ed. 2015, 54[doi]
- Aminoketyl Radicals in Organic Synthesis: Stereoselective Cyclization of 5- and 6-Membered Cyclic Imides to 2-Azabicycles using SmI2-H2O. Shi S.; Szostak, M. Org. Lett. 2015, 17, 5144 [doi]
- Sterically-Controlled Pd-Catalyzed Chemoselective Ketone Synthesis via N-C Cleavage in Twisted Amides. Meng, G.; Szostak, M. Org. Lett. 2015, 17 [doi]
- Recent Developments in the Synthesis and Reactivity of Isoxazoles: Metal Catalysis and Beyond.Hu, F.; Szostak, M. Adv. Synth. Catal. 2015, 357, 2583. [doi]
- Determination of Structures and Energetics of Small- and Medium-Sized One-Carbon Bridged Twisted Amides using ab Initio Molecular Orbital Methods. Implications for Amidic Resonance along the C-N Rotational Pathway. Szostak, R.; Aubé, J.; Szostak, M. J. Org. Chem. 2015, 80, 7905. [doi]
- An Efficient Computational Model to Predict Protonation at the Amide Nitrogen and Reactivity along the C-N Rotational Pathway. Szostak, R.; Aubé, J.; Szostak, M. Chem. Commun. 2015, 51, 6395.[doi]
- Pd-Catalyzed C-H Activation: Expanding the Portfolio of Metal-Catalyzed Functionalization of Unreactive C-H Bonds by Arene-Chromium π-Complexation. Hu, F.; Szostak, M. ChemCatChem2015, 7, 1061. [doi]
- Highly Chemoselective Reduction of Amides (Primary, Secondary and Tertiary) to Alcohols using SmI2/H2O/Amine under Mild Conditions. Szostak, M.; Spain, M.; Eberhart, A. J.; Procter, D. J. J. Am. Chem. Soc. 2014, 136, 2268. [doi]
- Substrate-Directable Electron Transfer Reactions. Dramatic Rate Enhancement in the Chemoselective Reduction of Cyclic Esters using SmI2-H2O: Mechanism, Scope and Synthetic Utility. Szostak, M.; Spain, M.; Choquette, K. A.; Flowers, R. A., II; Procter, D. J. J. Am. Chem. Soc.2013, 135, 15702. [doi]
- Selective Reduction of Barbituric Acids using SmI2-H2O: Synthesis, Reactivity and Structural Analysis of Tetrahedral Adducts. Szostak, M.; Spain, M.; Behlendorf, M; Procter, D. J. Angew. Chem. Int. Ed. 2013, 52, 12559. [doi]
- Non-Classical Lanthanide(II) Iodides: Uncovering the Importance of Proton Donors in TmI2-Promoted Electron Transfer. Facile C-N Bond Cleavage in Unactivated Amides. Szostak, M.; Spain, M.; Procter, D. J. Angew. Chem. Int. Ed. 2013, 52, 7237. [doi]
- Chemistry of Bridged Lactams and Related Heterocycles. Szostak, M.; Aubé, J. Chem. Rev. 2013,113, 5701. [doi]

GROUP
Prof. Michal Szostak
Assistant Professor
Ph.D., University of Kansas (2009) with Jeffrey Aubé
Postdoctoral, Princeton University (2010) with David MacMillan
Postdoctoral, University of Manchester (2011-2014) with David Procter
Postdoctoral Researchers
Dr. Feng Hu
Ph.D., Nanjing University, 2009 (Z. Huang)
Postdoctoral, Shanghai Institute of Materia Medica (Y. Hu)
Research Assistant Professor, SIOC (Q. Shen)
Postdoctoral, Lamar University (X. Lei)
Dr. Pradeep Nareddy
Ph.D., University of Geneva, 2013 (C. Mazet)
Postdoctoral, Leipzig University (C. Schneider)
Graduate Students
Shicheng Shi
M.S., SIOC, 2013 (R. Wang)
B.S., Nanjing Agriculture University, 2010
Guangrong Meng
M.S., Fudan University, 2014 (Q. Zhang)
B.S., Dalian Medical University, 2011
Chengwei Liu
M.S., Soochow University, 2014 (Y. Yao)
B.S., Zaozhuang University, 2011
Undergraduate Students
Syed Huq (Rutgers, Chemistry, 2014-present)
Marcel Achtenhagen (Rutgers, Chemistry, 2015-present)
Visiting Students
Yongmei Liu (Yangzhou University, R. Liu)
//////Nickel-Catalyzed, Decarbonylative Suzuki–Miyaura Coupling, Amides, Biaryls

 Highlights
Highlights