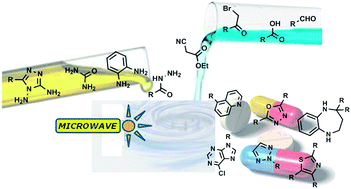
Green Chem., 2013, Advance Article
DOI: 10.1039/C3GC41431K, Paper
DOI: 10.1039/C3GC41431K, Paper
Roselinde Ooms, Michiel Dusselier, Jan A. Geboers, Beau Op de Beeck, Rick Verhaeven,
Elena Gobechiya, Johan A. Martens, Andreas Redl, Bert F. Sels
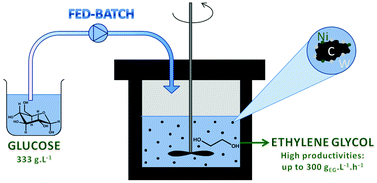
Fed-batch reactor technology was used for the highly productive conversion of
concentrated sugar solutions into ethylene glycol using bifunctional nickel tungsten
carbide catalysts.
Elena Gobechiya, Johan A. Martens, Andreas Redl, Bert F. Sels
Fed-batch reactor technology was used for the highly productive conversion of
concentrated sugar solutions into ethylene glycol using bifunctional nickel tungsten
carbide catalysts.