NEW DRUG APPROVALS ACHIEVES ONE LAKH VIEWS IN 166 COUNTRIES « New Drug Approvals:
'via Blog this'
DR ANTHONY MELVIN CRASTO Ph.D ( ICT, Mumbai) , INDIA 30 Yrs Exp. in the feld of Organic Chemistry. Serving chemists around the world. Helping them with websites on Chemistry.Millions of hits on google, world acclamation from industry, academia, drug authorities for websites, blogs and educational contributionn
Thursday 17 October 2013
Wednesday 16 October 2013
Microwave-assisted synthesis of N-heterocycles in medicinal chemistry
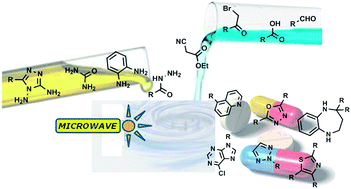
Med. Chem. Commun., 2013, 4,1323-1343
DOI: 10.1039/C3MD00152K, Review Article
DOI: 10.1039/C3MD00152K, Review Article
Davide Garella, Emily Borretto, Antonella Di Stilo, Katia Martina, Giancarlo Cravotto, Pedro Cintas
Microwave-assisted synthesis of heterocycle libraries has given an impressive contribution to drug discovery and development.http://pubs.rsc.org/en/Content/ArticleLanding/2013/MD/C3MD00152K?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FMD+%28RSC+-+Med.+Chem.+Commun.+latest+articles%29#!divAbstract
Microwave-assisted synthesis of heterocycle libraries has given an impressive contribution to drug discovery and development.http://pubs.rsc.org/en/Content/ArticleLanding/2013/MD/C3MD00152K?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FMD+%28RSC+-+Med.+Chem.+Commun.+latest+articles%29#!divAbstract
Microwave-assisted synthesis of N-heterocycles in medicinal chemistry
The syntheses of almost all N-heterocycles have now been successfully performed under microwave irradiation and have provided significant improvements in the reaction time and efficiency. The peculiar properties of dielectric heating give it the ability to strongly promote cyclocondensation, cycloaddition and selective N-heterocycle functionalisation and it has, therefore, very much caught the attention of the medicinal chemistry community. In this work, we present an overview of recent literature and technical advances in this research field with the aim of providing insight into the applications of microwave-assisted synthesis in the preparation of the main drug categories that contain N-heterocycle scaffolds.
Monday 14 October 2013
A Novel Solid-Phase Synthesis of Quinolines
Short Paper | Regular issue | Vol 85, No. 3, 2012, pp.667-676
Published online: 26th January, 2012

http://www.heterocycles.jp/newlibrary/payments/form/22246/fulltext
Published online: 26th January, 2012
DOI: 10.3987/COM-11-12411
■ A Novel Solid-Phase Synthesis of Quinolines
E Tang,* Deshou Mao, Wen Li, Zhangyong Gao, and Pengfei Yao
*School of Chemical Science and Technology, Yunnan University, No. 2 Green Lake North Road, Kunming 650091, China
Abstract
A method for synthesizing substituted-quinolines using TMSOTf-catalyzed polystyrene-supported succinimidyl selenide-induced intramolecular seleno-arylation of tethered alkenes as a key step has been developed. The catalytic process provides an efficient method for the stereoselective and regioselective synthesis of tetrahydroquinoline possessing a seleno-functionality, followed by deprotection of tosyl group and syn-elimination of selenoxides to provide quinolines in good yields and purities.
http://www.heterocycles.jp/newlibrary/payments/form/22246/fulltext
Efficient Synthesis of 2,4-Diarylquinolines via Fe(III) Trifluoroacetate Catalyzed Three-Component Reactions under Solvent-Free Conditions
Paper | Regular issue | Vol 85, No. 3, 2012, pp.639-649
Published online: 9th February, 2012

Published online: 9th February, 2012
DOI: 10.3987/COM-12-12433
■ Efficient Synthesis of 2,4-Diarylquinolines via Fe(III) Trifluoroacetate Catalyzed Three-Component Reactions under Solvent-Free Conditions
Min Zhang,* Ting Wang, Biao Xiong, Fengxia Yan, Xiaoting Wang, Yuqiang Ding, and Qijun Song
*School of Chemistry and Materials Engineering, Jiangnan University, 1800 Lihu Road, Wuxi, Jiangsu Province, 214122, China
Abstract
A convenient and efficient one-pot synthesis of 2,4-diarylquinolines is described by using Fe(CF3CO2)3 as a catalyst for the first time. In this method, three-component coupling of arylaldehydes, arylamines and arylacetylenes furnished the corresponding products in good to excellent yields. The work features the use of cheap and eco-friendly catalyst, excellent functional group tolerance and solvent-free conditions. A variety of new products were afforded effectively.
Friday 11 October 2013
Subscribe to:
Posts (Atom)
