DR ANTHONY MELVIN CRASTO Ph.D ( ICT, Mumbai) , INDIA 30 Yrs Exp. in the feld of Organic Chemistry. Serving chemists around the world. Helping them with websites on Chemistry.Millions of hits on google, world acclamation from industry, academia, drug authorities for websites, blogs and educational contributionn
Wednesday, 13 November 2013
Friday, 8 November 2013
Conversion of sugars to ethylene glycol with nickel tungsten carbide in a fed-batch reactor: high productivity and reaction network elucidation
Green Chem., 2013, Advance Article
DOI: 10.1039/C3GC41431K, Paper
DOI: 10.1039/C3GC41431K, Paper
Roselinde Ooms, Michiel Dusselier, Jan A. Geboers, Beau Op de Beeck, Rick Verhaeven,
Elena Gobechiya, Johan A. Martens, Andreas Redl, Bert F. Sels
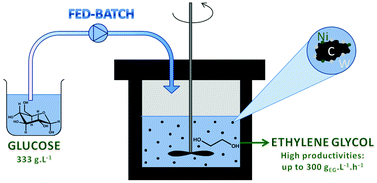
Fed-batch reactor technology was used for the highly productive conversion of
concentrated sugar solutions into ethylene glycol using bifunctional nickel tungsten
carbide catalysts.
Elena Gobechiya, Johan A. Martens, Andreas Redl, Bert F. Sels
Fed-batch reactor technology was used for the highly productive conversion of
concentrated sugar solutions into ethylene glycol using bifunctional nickel tungsten
carbide catalysts.
Conversion of sugars to ethylene glycol with nickel tungsten carbide
in a fed-batch reactor: high productivity and reaction
network elucidation
http://pubs.rsc.org/en/Content/ArticleLanding/2013/GC/C3GC41431K?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
Bifunctional nickel tungsten carbide catalysis was used for the conversion of aqueous sugar
solutions into short-chain polyols such as ethylene glycol. It is shown that very concentrated sugar
solutions, viz. up to 0.2 kg L−1, can be converted without loss of ethylene glycol selectivity
by gradually feeding the sugar solution. Detailed investigation of the reaction network
shows that, under the applied reaction conditions, glucose is converted via a retro-aldol
reaction into glycol aldehyde, which is further transformed into ethylene glycol by hydrogenation.
The main byproducts are sorbitol, erythritol, glycerol and 1,2-propanediol.
They are formed through a series of unwanted side reactions including
hydrogenation, isomerisation, hydrogenolysis and dehydration.
Hydrogenolysis of sorbitol is only a minor source of ethylene glycol. To assess the
relevance of the fed-batch system in biomass conversions, both the influence of the
catalyst composition and the reactor setup parameters like temperature, pressure
and glucose addition rate were optimized, culminating in ethylene glycol yields up to 66% and
separately, volume productivities of nearly 300 gEG L−1 h−1.
Saturday, 2 November 2013
World Drug Tracker: Gossypol a male antifertility agent with antispermatogenic activity and has been shown to contain anitumor, anitviral, and antioxidant properties
Wednesday, 30 October 2013
Tuesday, 29 October 2013
Synthesize 7-azanorbornane on an industrial scale
Synthesize 7-azanorbornane on an industrial scale
| Patent Number: | US 8404865 |
| Title: | Process for preparing azabicyclic compounds |
| Inventor(s): | Ambhaikar, Narendra Bhalchandra; Bear, Brian Richard; Fanning, Lev T. D.; Hughes, Robert; Littler, Benjamin |
| Patent Assignee(s): | Vertex Pharmaceuticals Incorporated, USA |
| Source: | U.S. Pat. Appl. Publ., 8pp. CODEN: USXXCO |
| Language: | English |
Abstract:The present invention relates to a process for prepg. azabicyclic compds. that are useful intermediates for synthesizing pharmaceutical compds. or salts thereof. Thus, azabicyclo[2.2.1]heptane hydrochloride (I·HCl) was prepd. from trans-4-aminocyclohexanol via N-protection with Boc2O in CH2Cl2 contg. Et3N; mesylation with MsCl in CH2Cl2 contg. Et3N; N-deprotection with CF3CO2H; cyclization with aq. NaOH; and treatment with
http://www.google.com.mx/patents/US8404865 view patent
7-azabicyclo[2.2.1]heptanes are useful intermediates in the synthesis of pharmaceutical compounds and salts thereof. For example, see U.S. Pat. Nos. 6,117,889 and 6,060,473, each of which is hereby incorporated by reference in its entirety
 | |
Despite the title of N. B. Ambhaikar and co-inventors’ patent, “Process for preparing azabicyclic compounds”, it only describes a process for preparing 7-azanorbornane (5) and its HCl salt (6). The inventors state that compound 5 is an intermediate in the synthesis of pharmaceutical compounds, but they do not mention any.
The patent’s examples describe the preparation of 5 and its precursors on a kilogram scale. The first step is protecting the amino group in 1 by converting it to tert-butoxycarbonyl (BOC) derivative 2 with the anhydride (BOC)2O in the presence of Na2CO3. The product is isolated in 88.8% yield. The reaction can also be carried out with K2CO3, but the yield is not reported.

In the second step, 2 is treated with methanesulfonyl chloride (MsCl) in the presence of Et3N to form methanesulfonate 3 in 96.6% isolated yield. In step three, the BOC group is removed by adding CF3CO2H in two batches. The product is amine salt 4. The recovered salt contains excess CF3CO2H; and as a result, the yield appears to be >100%.
In the final stage, the CF3CO2H salt is treated with NaOH to cyclize it to the desired compound. Azanorbornane 5 is recovered by fractional distillation; treating the fractions with concd HCl gives hydrochloride salt 6. The salt is recovered as a solid, dried, and recrystallized from MeOH and MeOH–THF. Although the examples contain significant detail, the product’s final yield and purity are not reported.
The process is an efficient method for preparing 7-azanorbornane and its salt. It is clearly suitable for large-scale production. (Vertex Pharmaceuticals [Cambridge, MA]. US Patent 8,404,865, March 26, 2013; Keith Turner)
NMR
7-azanorbornane HCl salt (6).
1HNMR (DMSO-d6) ppm 8.02-8.04 (d); 7.23-7.31 (m); 4.59 (s); 3.31 (s); 2.51-3.3 (m); 1.63-1.75 (m); 1.45-1.62 (m).
In one aspect, the invention includes a process for preparing Compound 7-azanorbornane
PL IGNORE NUMBER 7
- or a pharmaceutically acceptable salt thereof, comprising contacting trans-4-aminocyclohexanol with Boc anhydride to produce a compound of formula A
- contacting a compound of formula A with methanesulfonic acid to produce a compound of formula B
- contacting a compound of formula B with trifluoroacetic acid to produce a compound of formula C
- contacting a compound of formula C with hydroxide to produce a compound of formula
In some embodiments, the invention includes a method of producing a compound of formula 7-azanorbornane Hydrocloride salt
- The TFA salt of trans-4-aminocyclohexylmethanesulfonate (200 g, 650.9 mmol) was introduced into a 3-necked flask followed by the addition of water (2.200 L, 11 vol). NaOH (78.11 g, 1.953 mol, 3 eq) was slowly added, keeping the temperature of the reaction mixture below 25° C. and the mixture was stirred overnight. DCM (1.4 L, 7 vol) was then added and the mixture stirred, and the organic layer was separated. The aqueous layer was then extracted a second time with DCM (1.4 L, 7 vol), and the DCM layers were combined. HCl (108.5 mL, 12M, 1.3020 mol, 2 eq) was then added, the mixture was stirred for 30 min and then concentrated on a rotary evaporator to dryness. Acetonitrile (10 vol) was added and the mixture concentrated. This was repeated 3 times until all trace water was azeotropically removed, to provide 7-azabicyclo[2.2.1]heptane hydrochloride. The crude product was recrystallized from acetonitrile (10 vol) to provide 7-azabicyclo[2.2.1]heptane hydrochloride as a colorless crystalline solid.
Saturday, 26 October 2013
Subscribe to:
Comments (Atom)

 Asymmetric conjugate addition reactions of alkenylaluminums to
enones
Asymmetric conjugate addition reactions of alkenylaluminums to
enones 



