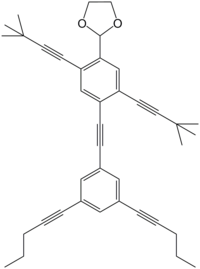
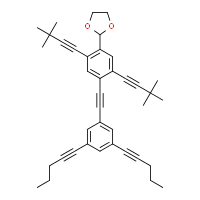
Systematic name
2-(4-{2-[3,5-bis(pent-1-yn-1-yl)phenyl]ethynyl}-2,5-bis(3,3-dimethylbut-1-yn-1-yl)phenyl)-1,3-dioxolane
Other names
1,3-dioxolane, 2-[2,5-bis(3,3-dimethyl-1-butyn-1-yl)-4-[2-(3,5-di-1-pentyn-1-ylphenyl)ethynyl]phenyl]
NanoKid
NanoPutian
618904-86-2 cas
NanoPutians are a series of organic molecules whose
structural formulae resemble human forms.
[1] James Tour et al. (
Rice University) designed and synthesized these compounds in 2003 as a part of a sequence of
chemical education for young students.
[2] The compounds consist of two
benzene rings connected via a few carbon atoms as the body, four
acetylene units each carrying an alkyl group at their ends which represents the hands and legs, and a
1,3-dioxolane ring as the head. Tour and his team at Rice University used the NanoPutians in their NanoKids educational outreach program. The goal of this program was to educate children in the sciences in an effective and enjoyable manner. They have made several videos featuring the NanoPutians as anthropomorphic animated characters.

Construction of the structures depends on
Sonogashira coupling and other synthetic techniques. By replacing the 1,3-dioxolane group with an appropriate ring structure, various other types of putians have been synthesized, e.g. NanoAthlete, NanoPilgrim, and NanoGreenBeret. Placing
thiol functional groups at the leg enables them to "stand" on a
gold surface.
Background
NanoKids Educational Outreach Program]
While there are no chemical uses for the NanoKid or any of its subsidiaries,
James Tour has turned the NanoKid into a lifelike character to educate children in the sciences. The goals of the outreach program, as described on the NanoKids website, are:
- “To significantly increase students’ comprehension of chemistry, physics, biology, and materials science at the molecular level."
- "To provide teachers with conceptual tools to teach nanoscale science and emerging molecular technology."
- "To demonstrate that art and science can combine to facilitate learning for students with diverse learning styles and interests."
- "To generate informed interest in nanotechnology that encourages participation in and funding for research in the field.”[3]
To accomplish these goals, several video clips, CDs, as well as interactive computer programs were created. Tour and his team invested over $250,000 into their project. In order to raise the funds for this endeavor, Tour used unrestricted funds from his professorship and small grants from Rice University, the
Welch Foundation, the nanotech firm
Zyvex, and
Texas A&M University. Tour also received $100,000 in 2002 from the Small Grants for Exploratory Research program, a division of the
National Science Foundation.
[4]
The main characters in the videos are animated versions of the NanoKid. They star in several videos and explain various scientific concepts, such as the
periodic table,
DNA, and
covalent bonding.
Rice conducted several studies into the effectiveness of using the NanoKids materials. These studies found mostly positive results for the use of the NanoKids in the classroom. A 2004-2005 study in two schools districts in Ohio and Kentucky found that using NanoKids led to a 10-59% increase in understanding of the material presented. Additionally, it was found that 82% of students found that NanoKids made learning science more interesting.
[5]

Synthesis of NanoKid
Upperbody of NanoKid
To create the first NanoPutian, dubbed the NanoKid,
1,4-dibromobenzene was
iodinated in
sulfuric acid. To this product, “arms”, or 3,3-Dimethylbutyne, were then added through
Sonogashira coupling. Formylation of this structure was then achieved through using the
organolithium reagent n-butyllithium followed by quenching with
N,N-dimethylformamide (DMF) to create the aldehyde. 1,2-Ethanediol was added to this structure to protect the aldehyde using
p-toluenesulfonic acid as a
catalyst. Originally, Chanteau and Tour aimed to couple this structure with alkynes, but this resulted in very low yields of the desired products. To remedy this, the
bromide was replaced with
iodide through lithium-halogen exchange and quenching by using 1,2-diiodoethane. This created the final structure of the upper body for the NanoKid.
[1]
Lowerbody of NanoKid
The synthesis of NanoPutian’s lower body begins with
nitroaniline as a starting material. Addition of Br
2 in
acetic acid places two equivalents of
bromine on the
benzene ring. NH
2 is an
electron donating group, and NO
2 is an
electron withdrawing group, which both direct bromination to the meta position relative to the NO
2 substituent. Addition of [[NaNO
2]], [[H
2SO
4]], and
EtOH removes the NH
2¬ substituent. The
Lewis acid SnCl
2, a
reducing agent in THF/EtOH solvent, replaces NO
2 with NH
2, which is subsequently replaced by iodine upon the addition of NaNO
2, H
2SO
4, and KI to yield 3,5-dibromoiodobenzene. In this step, the
Sandmeyer reaction converts the primary amino group (NH
2) to a diazonium leaving group (N
2), which is subsequently replaced by iodine. Iodine serves as an excellent coupling partner for the attachment of the stomach, which is executed through Sonogashira coupling with
trimethylsilylacetylene to yield 3,5-dibromo(trimethylsilylethynyl)benzene. Attachment of the legs replaces the Br substituents with 1-pentyne through another Sonogashira coupling to produce 3,5-(1′-Pentynyl)-1-(trimethylsilylethynyl) benzene. To complete the synthesis of the lower body, the TMS protecting group is removed by selective deprotection through the addition of K
2CO
3, MeOH, and CH
2Cl
2 to yield 3,5-(1′-Pentynyl)-1-ethynylbenzene.
[1]
Attachment of Upperbody to Lowerbody of NanoKid
Derivatives of NanoKid
Synthesis of NanoProfessionals[]
The series of NanoProfessionals were created using the NanoKid as the starting material. This was done by adding an excess amount of a 1,2- or 1,3- diol to the NanoKid in the presence of a catalytic amount of
p-toluenesulfonic acid and microwave oven-irradiation. The use of
microwave irradiation reduced the reaction times. These reactions resulted in an
acetal exchange, which changed the structure of the head of the NanoKid to create the different head structures of the NanoProfessionals, which include: NanoAthlete, NanoPilgrim, NanoGreenBeret, NanoJester, NanoMonarch, NanoTexan, NanoScholar, NanoBaker, and NanoChef. By creating a series of different figures, the ultimate product was a recognizably diverse population of NanoPutians.
[2]
Although the majority of the figures are depicted in their
equilibrium conformations, some of the NanoPutians include nonequilibrium conformations in order to make them more recognizable to nonchemists. Many liberties were taken in the visual depiction of the head dressings of the NanoPutians.
[2]
The entire population of NanoPutians (with the exception of the NanoChef) were generated in one microwave oven reaction and confirmed by
mass spectrometry and
1H
NMR.
[1]
Below is a table listing the diols needed to convert the NanoKid into various NanoProfessionals. The diols used to create NanoPilgrim and NanoTexan were made through reductive
pinacol coupling of the 1,4- and 1,5-diketones with SmI
2 and Mg/TiCl
4. To create the diols used to make the NanoMonarch and the NanoScholar, catalytic OsO
4 was used to dihydroxylate the respective cycloalkenes. The diastereomeric ratios were determined through
1H NMR using the diastereotopic acetal protons.
[1]
Synthesis of the NanoKid in Upright Form]

Stick Figure NanoPutian in its Energy Minimized Conformation. Determined Using Spartan.
3-Butyn-1-ol was reacted with
methanesulfonyl chloride and
triethanolamine to produce its
mesylate. The mesylate was displaced to make thiolacetate. The thiol was coupled with 3,5-dibromo(trimethylsilylethynyl)benzene to create a free alkyne. The resulting product, 3,5-(4’-thiolacetyl-1’-butynyl)-1-(trimethylsilylethynyl)-benzene, had its trimethylsilyl group removed using
tetra-n-butylammonium fluoride (TBAF) and AcOH/Ac
2O in THF. The free alkyne was then coupled with the upper body product from the earlier synthesis. This resulted in a NanoKid with protected thiol feet.
[1]
To make the NanoKid “stand’, the acetyl protecting groups were removed through the use of
ammonium hydroxide in THF to create the free thiols. A gold-plated substrate was then dipped into the solution and incubated for four days.
Ellipsometry was used to determine the resulting thickness of the compound, and it was determined that the NanoKid was upright on the substrate.
[1]
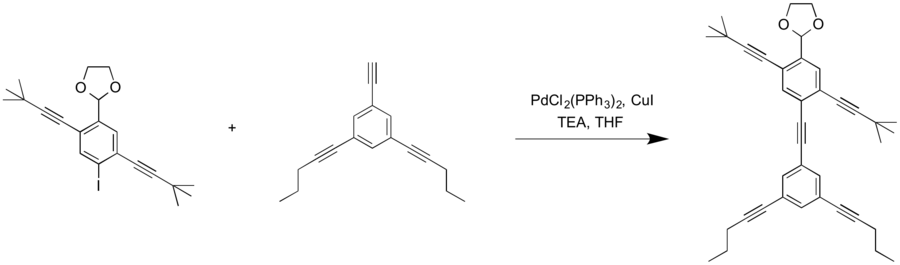
Synthesis of NanoPutian Chain
Synthesis of the upper part of the NanoPutian chain begins with 1,3-dibromo-2,4-diiodobenzene as the starting material. Sonogashira coupling with 4-oxytrimethylsilylbut-1-yne produces 2,5-bis(4-tert-butyldimethylsiloxy-1′-butynyl)-1,4-di-bromobenzene. One of the bromine substituents is converted to an aldehyde through an S
N2 reaction with the strong base, n-BuLi, and THF in the aprotic polar solvent, DMF to produce 2,5-bis(4-tert-butyldimethylsiloxy-1′-butynyl)-4-bromobenzaldehyde. Another Sonogashira coupling with 3,5-(1′-Pentynyl)-1-ethynylbenzene attaches the lower body of the NanoPutian. The conversion of the aldehyde group to a diether “head” occurs in two steps. The first step involves addition of ethylene glycol and
trimethylsilyl chloride (TMSCl) in CH
2Cl
2 solvent. Addition of TBAF in THF solvent removes the silyl protecting group.
[1]

References
- ^ a b c d e f g h i Chanteau, S. H.; Tour, J. M. (2003). "Synthesis of Anthropomorphic Molecules: The NanoPutians". The Journal of Organic Chemistry 68 (23): 8750–8766.doi:10.1021/jo0349227. PMID 14604341. edit
- ^ a b c Chanteau, S. H.; Ruths, T.; Tour, J. M. (2003). "Arts and Sciences Reunite in Nanoput: Communicating Synthesis and the Nanoscale to the Layperson". Journal of Chemical Education 80 (4): 395. doi:10.1021/ed080p395. edit
- ^ “Welcome to Nanokids.” Accessed May 6, 2013. http://nanokids.rice.edu/.
- ^ “C&EN: EDUCATION - ‘NANOKIDS’ TRY TO GET INTO MIDDLE SCHOOL.” Accessed May 10, 2013. http://pubs.acs.org/cen/education/8214/8214nanokids.html.
- ^ “NanoKids - Mission.” Accessed May 6, 2013. http://cohesion.rice.edu/naturalsciences/nanokids/mission.cfm?doc_id=3039.
External link
In 2003, there was a paper published which looked like it was going to be a good candidate for the Ig Nobel Prize. It was “Synthesis of Anthropomorphic Molecules: The NanoPutians” by Professor James Tour, a chemistry professor at Rice University’s Institute for Nanoscale Science and Technology. The word “NanoPutian” is a portmanteau of “nano”, which means a billionth and the “Lilliputian” from the novel Gulliver’s Travels.
The Tour group designed and synthesized a number of human-shaped organic molecules in this paper. Shown in Figure 1 is a molecule named NanoKid, which was chosen by the group as a basic skeleton. The 3-D model looks like the figure on the right and the structural formula used by chemists is shown on the left. The structural formula might look more human, since the oxygen atoms look kind of like the eyes.


Fig 1 NanoKid
The functional group used for the head part of NanoKid is called acetal. This group is easily exchangeable to make NanoPutians of various occupations (Figure 2). Let’s not be too picky about the bond angles of NanoMonarch and NanoTexan.

Fig 2 various NanoPutians
Unfortunately, NanoBalletDancer seems to be the only one having a different posture (Figure 3). Personally, I would be interested in making NanoPitcher or NanoGermanSuplex!

Fig 3 NanoBalletDancer
The Tour Group also synthesized NanoPutians standing on gold surface with thiol functional groups on their feet, a NanoPutian couple dancing (Figure 4), and even a polymer of NanoPutians (Figure 5).

Fig 4 NanoPutian Couple

Fog 5 NanoPutian polymer
NanoPutians aren’t actually the first example of human-shaped molecule. For example, the molecule shown in Figure 6 has appeared as a joke in a journal published on April Fool’s Day. The molecule shown in Figure 7 has been introduced once as Buddha molecule. Nevertheless, NanoPutians were probably the first case where human-shaped molecules were synthesized systematically(?) to be published as a full paper.


Fig 6 human-shaped molecule Fig 7 molecular Buddha
The Role of NanoPutians
Besides being human-shaped, the NanoPutian molecules have neither notable properties nor potential usefulness for future. The synthesis is also too straightforward to make any significant methodological contribution to chemical science.
Then how did this research get funded and get to be published on Journal of Organic Chemistry? It turns out that the synthesis was a part of the chemistry education program at Rice University aimed at introducing nanotechnology to young students. In fact, it has also been on the cover page of Journal of Chemical Education too. It’s funny though, to imagine the faces of the journal editors when they first read the paper.
But come to think of it, molecules like dodecahedrane and kekulene might not be so different in terms of not having much to appeal other than their structural beauty. Even “total synthesis of biologically active natural products”, the most respected subfield of organic chemistry, has been criticized on its meaning recently. In a way, the NanoPutian research seems to me as a voice saying “synthetic targets should be selected more freely” and almost as an antithesis against the state of organic chemistry today.
Anyway, this paper was introduced by general media and was also one of the topics that received most feedbacks on my homepage. There were those who dismissed it as a meaningless play by chemists, but in terms of directing public interest toward organic chemistry wasn’t it a hundred times more effective than ordinary researches? I think it was an excellent work for the education of young chemists as well.
Professor Tour’s playful sense of molecular design can be seen in his research of NanoCars too, which I will introduce in a separate column. This is a wonderful work which can impress both serious scientists and general public.
 nanohippie
nanohippie
 nanorobot
nanorobot
NanoSports:
Nano’Tainment:


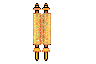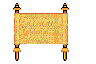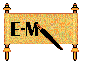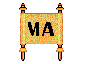





























 The enzyme cyclic GMP-AMP synthase stimulates the immune response during HIV infections
The enzyme cyclic GMP-AMP synthase stimulates the immune response during HIV infections A palmitoyl thioesterase that decreases the ability of Toxoplasma gondiito invade host cells has been identified
A palmitoyl thioesterase that decreases the ability of Toxoplasma gondiito invade host cells has been identified Photodecomposition of tetrachloroethylene provides a safer source of small chlorinated building blocks for organic synthesis
Photodecomposition of tetrachloroethylene provides a safer source of small chlorinated building blocks for organic synthesis