

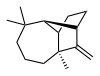
Longifolene is the common (or trivial) chemical name of a naturally occurring, oily liquid hydrocarbon found primarily in the high-boiling fraction of certain pine resins. The name is derived from that of a pine species from which the compound was isolated,[1] Pinus longifolia (obsolete name for Pinus roxburghii Sarg.)[2]
Chemically, longifolene is a tricyclic sesquiterpene. This molecule is chiral, and the enantiomer commonly found in pines and other higher plants exhibits a positive optical rotation of +42.73°. The other enantiomer (optical rotation −42.73°) is found in small amounts in certain fungi and liverworts.
Longifolene is used in organic synthesis for the preparation of dilongifolylborane,[3] a chiral hydroborating agent.
Longifolene is also one of two most abundant aroma constituents of lapsang souchong tea, because the tea is smoked over pine Due to the compact tricyclic structure and lack of functional groups, Longifolene is an attractive target for research groups highlighting new synthetic methodologies. Notable syntheses are by Corey,[5][6] McMurray,[7] Johnson,[8] Oppolzer,[9] and Schultz.[10]

Longifolene total synthesis by Corey
| Author | Elias J. Corey |
|---|---|
| Publication year | 1961 |
| Synthesis type | Total synthesis |
| Number of steps | 14 (linear) |
| References |
http://www.synarchive.com/syn/118
............
Total synthesis of Longifolene:
| Reference: | Corey, E. J.; Ohno, M.; Mitra, R. B.; Vatakencherry, P. A. J. Am. Chem. Soc. 1964, 86, 478. DOI |
| Keywords: | Ketone → Ketal • CompE+-Ketone/Ketone+glycol • O-H → O-SO2R • Ketone → Ketal(thio) • Ketone → Alkyl-OH • Alkyl-OH → Ketone • Li-Me+Ketone • Ketone+Li-Alkyl • Dehydration → Ene • Wittig-alkyl+Ketone • Alkene → Diol-1,2 • CompNu-Alcohol/Alcohol+RSO2Cl • Pinacol • ConjAdd Enolate • Ketone enolate+Enone • Hydrogenolysis C-S • Ketone → CH2 • |
| Reagents: | Wieland-Miescher • Glycol • TsOH • PPh3=CH-Me • OsO4 • TsCl, Py • LiClO4 • Carbonate, calcium • HCl, H2O • NEt3 • NaCPh3 • MeI • Thiol, (CH2)2-SH • BF3·OEt2 • AlH4-Li+ • Hydrazine • CrO3 • MeLi • SOCl2 • |
 |
|||||||||||||||||||||||||||||
BiosynthesisThe biosynthesis of longifolene begins with farnesyl diphosphate (1) (also called farnesyl pyrophosphate) by means of a cationic polycyclization cascade. Loss of the pyrophosphate group and cyclization by the distal alkene gives intermediate 3, which by means of a 1,3-hydride shift gives intermediate 4. After two additional cyclizations, intermediate 6 produces longifolene by a 1,2-alkyl migration. |
|||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
1,4-Methanoazulene, Junipen, (+)-Longifolene, 475-20-7, 3,3,7-trimethyl-8-methylenetricyclo[5.4.0.02,9]undecane, Kuromatsuen, Kuromatsuene
Molecular Formula: C15H24 Molecular Weight: 204.35106
....................
The borane derivative dilongifolylborane is used in organic synthesis as a chiral hydroborating agent.[12]
- Naffa, P.; Ourisson, G. Bulletin de la Société chimique de France, 1954, 1410.
- Simonsen, J. L. J. Chem. Soc. 1920, 117, 570.
- Jadhav, P. K.; Brown, H. C. J. Org. Chem. 1981, 46, 2988.
- Shan-Shan Yao; Wen-Fei Guo; YI Lu; Yuan-Xun Jiang, "Flavor Characteristics of Lapsang Souchong and Smoked Lapsang Souchong,a Special Chinese Black Tea with Pine Smoking Process", Journal of Agricultural and Food Chemistry, Vol. 53, No.22, (2005)
- Corey, E. J. et al. J. Am. Chem. Soc. 1961, 83, 1251.
- Corey, E. J. et al. J. Am. Chem. Soc. 1964, 86, 478.
- McMurray, J. E.; Isser, S. J. J. Am. Chem. Soc. 1972, 94, 7132.
- Volkermann, R. A.; Andrews, G. C.; Johnson, W. S. J. Am. Chem. Soc. 1975, 97, 4777-4779.
- Oppolzer, W.; Godel, T. J. Am. Chem. Soc. 1978, 100, 2583.
- Schultz, A. G. et al. J. Org. Chem. 1985, 50, 915.
- Ho, Gregory J. Org. Chem. 2005, 70, 5139 -5143.
- Dev, Sukh (1981). "Aspects of longifolene chemistry. An example of another facet of natural products chemistry". Accounts of Chemical Research 14 (3): 82–88. doi:10.1021/ar00063a004.


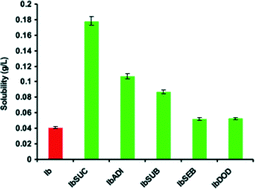

 BRAZIL WORLDCUP WEEK 2014
BRAZIL WORLDCUP WEEK 2014



 A route to oxygenated pyrano[3,2-a]- and
pyrano[2,3-a]carbazole alkaloids
A route to oxygenated pyrano[3,2-a]- and
pyrano[2,3-a]carbazole alkaloids A mild and catalytic methylation of amines with formic acid
A mild and catalytic methylation of amines with formic acid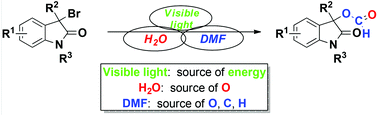



 New carbooxygenation-type version of the Meerwein arylation
allows introduction of oxygen from air by using manganese dioxide
New carbooxygenation-type version of the Meerwein arylation
allows introduction of oxygen from air by using manganese dioxide