Microwave flow reactor for the Bohlmann–Rahtz synthesis of pyridine 2b.
read at
http://www.beilstein-journals.org/bjoc/single/articleFullText.htm?publicId=1860-5397-9-232
Microwave flow reactor for the Bohlmann–Rahtz synthesis of pyridine 2b.
read at
http://www.beilstein-journals.org/bjoc/single/articleFullText.htm?publicId=1860-5397-9-232
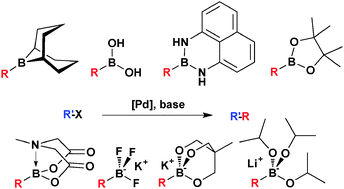
The Bohlmann–Rahtz pyridine synthesis and the Hantzsch dihydropyridine synthesis can be carried out in a microwave flow reactor or using a conductive heating flow platform for the continuous processing of material.
In the Bohlmann–Rahtz reaction, the use of a Brønsted acid catalyst allows Michael addition and cyclodehydration to be carried out in a single step without isolation of intermediates to give the corresponding trisubstituted pyridine as a single regioisomer in good yield.
Furthermore, 3-substituted propargyl aldehydes undergo Hantzsch dihydropyridine synthesis in preference to Bohlmann–Rahtz reaction in a very high yielding process that is readily transferred to continuous flow processing.
Mark C. Bagley1
,
Vincenzo Fusillo2,
Robert L. Jenkins2,
M. Caterina Lubinu2 and
Christopher Mason3
1Department of Chemistry, School of Life Sciences, University of Sussex, Falmer, Brighton, East Sussex, BN1 9QJ, UK
2School of Chemistry, Main Building, Cardiff University, Park Place, Cardiff, CF10 3AT, UK
3CEM Microwave Technology Ltd, 2 Middle Slade, Buckingham, MK18 1WA, UK
This article is part of the Thematic Series "Chemistry in flow systems III".
Guest Editor: A. Kirschning
Beilstein J. Org. Chem. 2013, 9, 1957–1968.


 ChemDraw is a leading chemistry molecules drawing software. ChemDraw is easy to use and best software for drawing a molecule structures and finding stereochemistry of the molecules.
ChemDraw is a leading chemistry molecules drawing software. ChemDraw is easy to use and best software for drawing a molecule structures and finding stereochemistry of the molecules.











 A magnetic resonance (MR) probe can be used to distinguish old and new blood clots
A magnetic resonance (MR) probe can be used to distinguish old and new blood clots










 A series of organic conjugated molecules containing 2,3-benzopyridiazine and thiophene derivatives has been synthesized
A series of organic conjugated molecules containing 2,3-benzopyridiazine and thiophene derivatives has been synthesized A rhodium-based catalyst reverses regioselectivity in the production of hydrazones
A rhodium-based catalyst reverses regioselectivity in the production of hydrazones