Revealing the Essentials for Effective β-Glucopyranoside Recognition

A step forward in the design of synthetic receptors for the recognition of carbohydrates
Read more

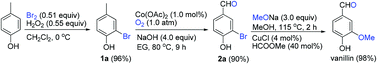
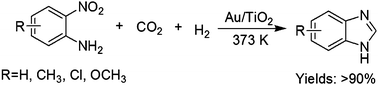

/V.Eifler-Lima/Scheme1.gif)
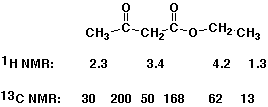
 14 and 24 represent relatively simple methyl groups; the triplets at
14 and 24 represent relatively simple methyl groups; the triplets at  59 and 47 represent a CH2 groups bonded to mildly electronegative groups; the singlets at
59 and 47 represent a CH2 groups bonded to mildly electronegative groups; the singlets at  207 and 172 are in the carbonyl region, and most likely a ketone or aldehyde (
207 and 172 are in the carbonyl region, and most likely a ketone or aldehyde ( 207) and an ester (
207) and an ester ( 172).
172). 1670 cm-1). The minor peak at 3400 cm-1 is too small to be an -OH.
1670 cm-1). The minor peak at 3400 cm-1 is too small to be an -OH. 4.1. The two singlets at
4.1. The two singlets at  2.2 and 3.2 suggest isolated CH2 and CH3 groups and the CH2 must be adjacent to one or more electronegative groups.
2.2 and 3.2 suggest isolated CH2 and CH3 groups and the CH2 must be adjacent to one or more electronegative groups.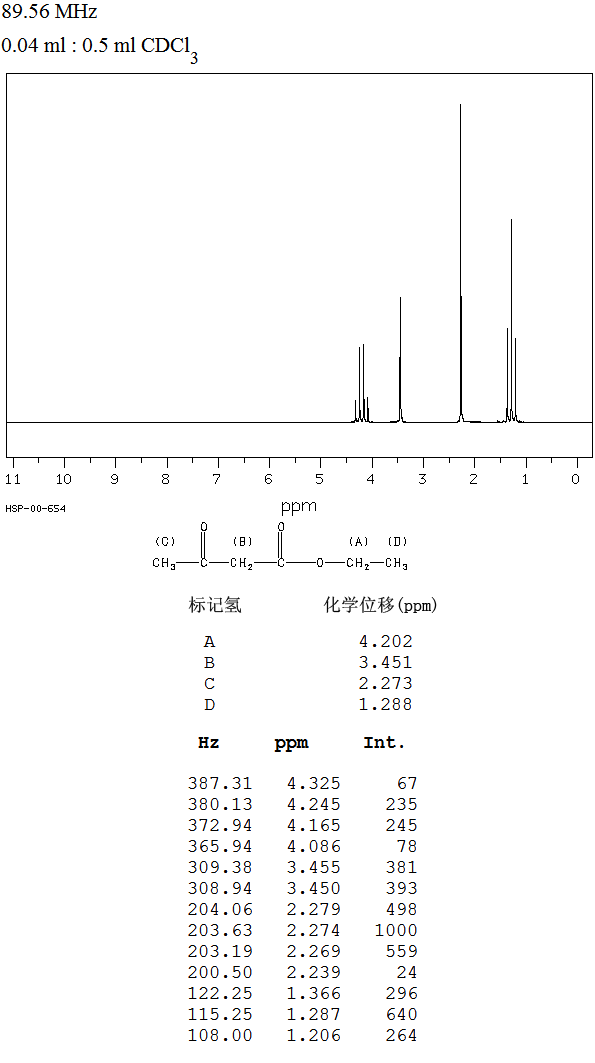
 14 and 24 represent relatively simple methyl groups; the triplets at
14 and 24 represent relatively simple methyl groups; the triplets at  59 and 47 represent a CH2 groups bonded to mildly electronegative groups; the singlets at
59 and 47 represent a CH2 groups bonded to mildly electronegative groups; the singlets at  207 and 172 are in the carbonyl region, and most likely a ketone or aldehyde (
207 and 172 are in the carbonyl region, and most likely a ketone or aldehyde ( 207) and an ester (
207) and an ester ( 172).
172).


 O). The spectrum is consistent with a molecule which can lose methyl or ethoxy radicals, or can undergo fragmentation to form the acylium radical cation.
O). The spectrum is consistent with a molecule which can lose methyl or ethoxy radicals, or can undergo fragmentation to form the acylium radical cation.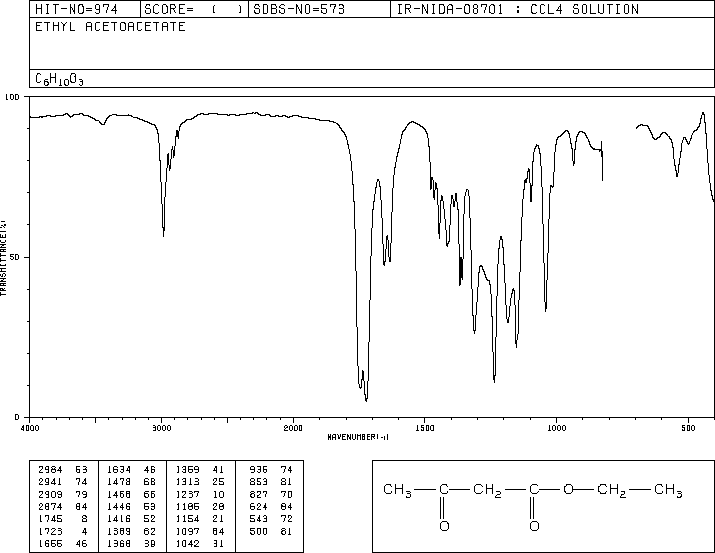

 1D DEPT135
1D DEPT135 2D [1H,13C]-HSQC
2D [1H,13C]-HSQC 2D [1H,13C]-HMBC
2D [1H,13C]-HMBC 2D [1H,1H]-COSY
2D [1H,1H]-COSY 2D [1H,13C]-HMQC
2D [1H,13C]-HMQC