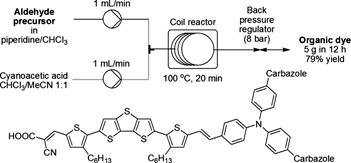
Iron-Catalyzed Synthesis of 2-Arylbenzo[b]furans
Synthetic Communications
Volume 43, Issue 6, 2013
Jianguo Yang, Guodong Shen & Dingben Chen
pages 837-847
DOI:10.1080/00397911.2011.610550
http://www.tandfonline.com/doi/abs/10.1080/00397911.2011.610550
copy paste link in browser
Abstract
An iron-catalyzed procedure was employed to achieve both the Sonogashira cross-coupling and intramolecular o-arylation of o-iodophenols and aryl acetylenes/1-substituted-2-trimethylsilyl acetylenes. A variety of 2-arylbenzo[b]furans were synthesized in moderate to good yields under the catalysis of 5% FeCl3 and 10% 1,10-phenanthroline.