Catal. Sci. Technol., 2017, Advance Article
DOI: 10.1039/C7CY01621B, Paper
DOI: 10.1039/C7CY01621B, Paper
Youming Ni, Lei Shi, Hongchao Liu, Wenna Zhang, Yong Liu, Wenliang Zhu, Zhongmin Liu
Halide-free and noble metal-free pyridine-modified H-mordenites exhibit high stability and selectivity in methanol carbonylation to acetic acid.
Halide-free and noble metal-free pyridine-modified H-mordenites exhibit high stability and selectivity in methanol carbonylation to acetic acid.
A green route for methanol carbonylation
Abstract
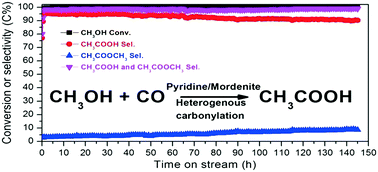
Acetic acid is one of the most important bulk commodity chemicals and is currently manufactured by methanol carbonylation reactions with rhodium or iridium organometallic complexes and halide-containing promoters named Monsanto or BP Cativa™ homogeneous processes, respectively. Developing a halide-free catalyst and a heterogeneous process for methanol carbonylation is of great importance and has recently attracted extensive research attention. Here, we report a green route for direct synthesis of acetic acid via vapor-phase carbonylation of methanol with a stable, selective, halide-free, and noble metal-free catalyst based on pyridine-modified H-mordenite zeolite. Methanol conversion and acetic acid selectivity can reach up to 100% and 95%, respectively. Only little deactivation is observed during the 145 hour reaction.
////////////