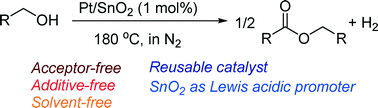
Catal. Sci. Technol., 2014, 4,3631-3635
DOI: 10.1039/C4CY00979G, Communication
DOI: 10.1039/C4CY00979G, Communication
Sondomoyee Konika Moromi, S. M. A. Hakim Siddiki, Md. Ayub Ali, Kenichi Kon, Ken-ichi Shimizu
Pt/SnO2 is presented as the first example of a reusable heterogeneous catalyst for acceptorless dehydrogenative coupling of primary alcohols to esters under additive-free and solvent-free conditions.
Supported platinum catalysts have been studied for the acceptor-free dehydrogenative
coupling of primary alcohols to esters in the liquid phase under solvent-free conditions in N2 at 180 °C.
The activity depends on the support material, and Pt-loaded SnO2 (Pt/SnO2) gives the highest activity. Pt/SnO2 shows higher activity than various transition metals
(Ir, Re, Ru, Rh, Pd, Ag, Co, Ni, Cu) loaded on SnO2. The Pt/SnO2
catalyst (1 mol%) selectively converted various primary alcohols to their corresponding esters in moderate to
high isolated yield (53–91%). This is the first example of reusable heterogeneous catalysts for the acceptor-free dehydrogenative coupling of primary alcohols to esters
under additive-free and solvent-free conditions. Mechanistic and infrared
(IR) studies are also shown to discuss the reaction pathway and a possible role of the SnO2 support as Lewis acid sites that activate carbonyl groups of adsorbed aldehyde intermediates.
DOI: 10.1039/C4CY00979G
read
Pt/SnO2 is presented as the first example of a reusable heterogeneous catalyst for acceptorless dehydrogenative coupling of primary alcohols to esters under additive-free and solvent-free conditions.
Supported platinum catalysts have been studied for the acceptor-free dehydrogenative
coupling of primary alcohols to esters in the liquid phase under solvent-free conditions in N2 at 180 °C.
The activity depends on the support material, and Pt-loaded SnO2 (Pt/SnO2) gives the highest activity. Pt/SnO2 shows higher activity than various transition metals
(Ir, Re, Ru, Rh, Pd, Ag, Co, Ni, Cu) loaded on SnO2. The Pt/SnO2
catalyst (1 mol%) selectively converted various primary alcohols to their corresponding esters in moderate to
high isolated yield (53–91%). This is the first example of reusable heterogeneous catalysts for the acceptor-free dehydrogenative coupling of primary alcohols to esters
under additive-free and solvent-free conditions. Mechanistic and infrared
(IR) studies are also shown to discuss the reaction pathway and a possible role of the SnO2 support as Lewis acid sites that activate carbonyl groups of adsorbed aldehyde intermediates.
Acceptorless dehydrogenative coupling of primary alcohols to esters by heterogeneous Pt catalysts
Sondomoyee Konika Moromi,a S. M. A. Hakim Siddiki,b Md. Ayub Ali,a Kenichi Kona and Ken-ichi Shimizu*ab
*
Corresponding authors
a
Catalysis Research Center, Hokkaido University, N-21, W-10, Sapporo 001-0021, Japan
E-mail: kshimizu@cat.hokudai.ac.jp;
Fax: +81 11 706 9163
E-mail: kshimizu@cat.hokudai.ac.jp;
Fax: +81 11 706 9163
b
Elements Strategy Initiative for Catalysts and Batteries, Kyoto University, Katsura, Kyoto 615-8520, Japan
Catal. Sci. Technol., 2014,4, 3631-3635
DOI: 10.1039/C4CY00979G
read





 Benzo[g]coumarins most suitable for applications as photonic
materials
Benzo[g]coumarins most suitable for applications as photonic
materials Regio- and stereoselective synthesis of bispirooxindole
derivatives via a three-component 1,3-dipolar cycloaddition
Regio- and stereoselective synthesis of bispirooxindole
derivatives via a three-component 1,3-dipolar cycloaddition


![7,7-Dichlorobicyclo[4.1.0]heptane 7,7-Dichlorobicyclo[4.1.0]heptane](http://kriemhild.uft.uni-bremen.de/nop/img/chents/small/91_en.gif)

 Operating scheme
Operating scheme