Icatibant (trade name Firazyr) is a peptidomimetic drug consisting of ten amino acids, which is a selective and specific antagonist of bradykinin B2 receptors. It has been approved by the European Commission for the symptomatic treatment of acute attacks,[1][2] of hereditary angioedema (HAE) in adults (with C1-esterase-inhibitor deficiency).
Bradykinin is a peptide-based hormone that is formed locally in tissues, very often in response to a trauma. It increases vessel permeability, dilates blood vessels
and causes smooth muscle cells to contract . Bradykinin plays an
important role as the mediator of pain. Surplus bradykinin is
responsible for the typical symptoms of inflammation, such as swelling,
redness, overheating and pain. These symptoms are mediated by activation of
bradykinin B2 receptors. Icatibant acts as a bradykinin inhibitor by blocking
the binding of native bradykinin to the bradykinin B2 receptor.
Icatibant has received orphan drug status
in Australia, EU, Switzerland and US.
In the EU, the approval by
the European Commission (July 2008) allows Jerini to market Firazyr in the European
Union's 27 member states, as well as Switzerland, Lichtenstein and Iceland,
making it the first product to be approved in all EU countries for the
treatment of HAE.[1] In the US, the drug was granted FDA
approval on August 25, 2011.[3]
1.
1 "Jerini Receives European
Commission Approval for Firazyr (Icatibant) in the Treatment of HAE" (Press
release). Jerini AG. 2008-07-15.
Retrieved 2008-07-22.
2.
2 "Jerini Receives Positive CHMP
Opinion Recommending European Approval for Icatibant in the Treatment of HAE;
FDA Issues Not Approvable Letter" (Press release).Jerini AG. 2008-04-24. Retrieved 2008-07-22.
3.
3 FDA Approves Shire’s FIRAZYR
(icatibant injection) for Acute Attacks of Hereditary Angioedema (HAE)" (Press
release). Shire. Retrieved 2011-08-28.
On the August 25th 2011, the FDA approved Icatibant (trade name: FirazyrTM), a bradykinin B2 receptor (B2R) antagonist indicated for the treatment of acute attacks of hereditary angioedema (HAE) in patients aged 18 or older.
HAE is a rare genetic disease and is caused by low
levels of C1-esterase inhibitor (C1-INH), the major endogenous inhibitor
and regulator of the protease plasma kallikrein and the key regulator
of the Factor XII/kallikrein cascade. One component this cascade is
the production of bradykinin by
plasma kallikrein. During HAE attacks, disregulated activity of plasma
kallikrein leads to excessive bradykinin production; bradykinin is a potent
vasodilator, which s thought to be responsible for the characteristic HAE
symptoms of localised swelling, inflammation and pain.
Icatibant treats the clinical symptoms of HAE
attack by selective- and competitively binding, as an antagonist, to the B2
bradykinin receptor (B2R) , with similar affinity to bradykinin (1-10 nM for
the B2R, while affinity for the B1R is 100-fold lower). Icatibant is the first
in class agent against this target. The -tibant stem covers bradykinin
antagonists.
B2R is a Rhodopsin-like receptor, 391 amino
acid long, which belongs to the G protein-coupled receptor (GPCR) A3
family and is encoded by the BDKRB2 gene in humans. The amino acid
sequence of B2R is:
>B2R
MFSPWKISMFLSVREDSVPTTASFSADMLNVTLQGPTLNGTFAQSKCPQVEWLGWLNTIQ
PPFLWVLFVLATLENIFVLSVFCLHKSSCTVAEIYLGNLAAADLILACGLPFWAITISNN
FDWLFGETLCRVVNAIISMNLYSSICFLMLVSIDRYLALVKTMSMGRMRGVRWAKLYSLV
IWGCTLLLSSPMLVFRTMKEYSDEGHNVTACVISYPSLIWEVFTNMLLNVVGFLLPLSVI
TFCTMQIMQVLRNNEMQKFKEIQTERRATVLVLVVLLLFIICWLPFQISTFLDTLHRLGI
LSSCQDERIIDVITQIASFMAYSNSCLNPLVYVIVGKRFRKKSWEVYQGVCQKGGCRSEP
IQMENSMGTLRTSISVERQIHKLQDWAGSRQ



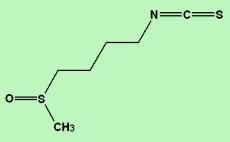
 1-Isothiocyanato-4-methylsulfinylbutane
1-Isothiocyanato-4-methylsulfinylbutane



 links
links