Progress in Microwave-Aided Chemical Synthesis
LINK IS
http://www.publish.csiro.au/paper/CH12366.htm
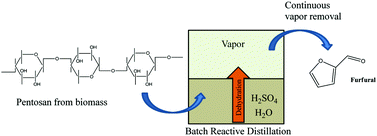
The continuing use of microwave (µwave) energy in chemical synthesis has been impressive over the past decade, with many reports incorporating µwave-based reactions. Two of the major benefits of using µwave heating are the remarkable decrease in reaction times and often high yield of products in comparison with classical heating, an ideal technology for synthetic chemists. Herein, we highlight some exciting examples of its recent utility in organic, medicinal, and natural product synthetic endeavours.
LINK IS
http://www.publish.csiro.au/paper/CH12366.htm
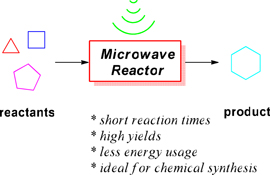
The continuing use of microwave (µwave) energy in chemical synthesis has been impressive over the past decade, with many reports incorporating µwave-based reactions. Two of the major benefits of using µwave heating are the remarkable decrease in reaction times and often high yield of products in comparison with classical heating, an ideal technology for synthetic chemists. Herein, we highlight some exciting examples of its recent utility in organic, medicinal, and natural product synthetic endeavours.