a
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, P. R. China
E-mail: masm@sioc.ac.cn
E-mail: masm@sioc.ac.cn
b
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, P. R. China
Org. Chem. Front., 2015,2, 470-475
DOI: 10.1039/C5QO00047E, http://pubs.rsc.org/en/Content/ArticleLanding/2015/QO/C5QO00047E#!divAbstract
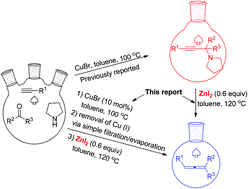
Zinc diiodide has been identified as an effective reagent for the efficient synthesis of trisubstituted allenes from propargylic amines. Compared to CdI2 this protocol offers a green approach. Due to the easy preparation of propargylic amines through the method developed by this group, this method provides a two-step synthesis of trisubstituted allenes from 1-alkynes, ketones, and pyrrolidine. Finally, an efficient synthesis of such trisubstituted allenes from 1-alkynes, ketones, and pyrrolidine via simple filtration has been developed. Compared with the CdI2-mediated protocol, the current protocol enjoys a much wider scope for ketones and affords functionalized allenes without further cyclization in some substrates.

No comments:
Post a Comment