Green Chem., 2014, Advance Article
DOI: 10.1039/C4GC00153B, Paper
DOI: 10.1039/C4GC00153B, Paper
Leiduan Hao, Yanfei Zhao, Bo Yu, Hongye Zhang, Huanjun Xu, Zhimin Liu
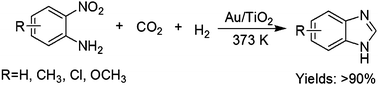
The gold-catalyzed synthesis of benzimidazoles from 2-nitroanilines and CO2 in the presence of H2 was reported, and a series of benzimidazoles were obtained under relatively mild conditions.
The gold-catalyzed synthesis of benzimidazoles from 2-nitroanilines and CO2 in the presence of H2 was reported, and a series of benzimidazoles were obtained under relatively mild conditions.
The gold-catalyzed synthesis of benzimidazoles from 2-nitroanilines and CO2 in the presence of
H2 was reported, and a series of benzimidazoles were obtained under relatively mild conditions.
Several supported Au catalysts including Au/TiO2, Au/Al2O3, Au/ZnO, Au/polyurea and Au/hydrotalcite
were examined for the synthesis of benzimidazole from the reaction of 2-nitroaniline with CO2 and H2,
among which Au/TiO2 displayed the best performance. The reaction mechanism was investigated,
and it was found that the production of benzimidazole underwent the formation of o-phenylenediamine
via the hydrogenation of 2-nitroaniline, followed by the cyclization of o-phenylenediamine with CO2 and H2.
This work provides a CO2-involved route for the synthesis of benzimidazoles,
which may widen the applications of CO2 in the chemical synthesis.

No comments:
Post a Comment