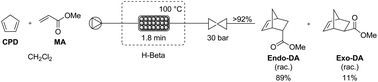
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC02556K, Paper
Valentin A. Rassadin, Dmitry P. Zimin, Gulnara Z. Raskil'dina, Alexander Yu. Ivanov, Vadim P. Boyarskiy, Semen S. Zlotskii, Vadim Yu. Kukushkin
A solvent- and halide-free atom-economical synthesis of practically useful pyridine-2-yl substituted ureas utilizes pyridine N-oxides and dialkylcyanamides.
Solvent- and halide-free synthesis of pyridine-2-yl substituted ureas through facile C–H functionalization of pyridine N-oxides
b
Ufa State Petroleum Technological University, Kosmonavtov 1, Ufa, Bashkortostan, Russia
c
Research Park SPbSU, Center for Magnetic Resonance, Saint Petersburg State University, Universitetskaya Nab. 7/9, 199034 Saint Petersburg, Russia
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC02556K
A novel solvent- and halide-free atom-economical synthesis of practically useful pyridine-2-yl substituted ureas utilizes easily accessible or commercially available pyridine N-oxides (PyO) and dialkylcyanamides. The observed C–H functionalization of PyO is suitable for the good-to-high yielding synthesis of a wide range of pyridine-2-yl substituted ureas featuring electron donating and electron withdrawing, sensitive, or even fugitive functional groups at any position of the pyridine ring (63–92%; 19 examples). In the cases of 3-substituted PyO, the C–H functionalization occurs regioselectively providing a route for facile generation of ureas bearing a 5-substituted pyridine-2-yl moiety.
1,1-Dimethyl-3-(pyridin-2-yl)urea
1,1-Dimethyl-3-(pyridin-2-yl)urea (4a)3
: From pyridine 1-oxide (1a) (95.0 mg, 1.00
mmol) and dimethylcyanamide (2a) (105 mg, 1.50 mmol), compound 4a (147 mg,
89%) was obtained according to GP1 as a yellow oil, which was then crystalized in
the freezer to give pale yellow solid, m.p. = 42.6–43.5 °C, lit.4 m.p. = 44–47 °C (EtOAc/hexane), Rf
= 0.25 (EtOAc). 1H NMR (400 MHz, CDCl3): δ = 3.00 (s, 6 H, NCH3), 6.88 (ddd, J = 7.3, 5.0, 0.9
Hz, 1 H), 7.30 (br. s, 1 H), 7.60 (ddd, J = 8.5, 7.3, 1.9 Hz, 1 H), 8.02 (dt, J = 8.5, 0.9 Hz, 1 H), 8.14
(ddd, J = 5.0, 1.9, 0.9 Hz, 1 H) ppm. 13C NMR (101 MHz, CDCl3): δ = 36.3 (2 С, CH3), 113.0 (CH),
118.1 (CH), 138.0 (CH), 147.3 (CH), 152.8 (C), 154.8 (C) ppm. NMR data are consistent with
previously reported.3 HRMS (ESI), m/z: [M + H]+ calcd. for C8H12N3O+
: 166.0975; found: 166.0977.
////////////